Science, Art, Litt, Science based Art & Science Communication
Comedian Robin Williams had been diagnosed with several health conditions before he committed suicide last year, according to his widow. He was "just disintegrating" physically and mentally in the months before his death. Williams died in August 2014 at age 63 and had been diagnosed with Parkinson's Disease three months prior. He was showing symptoms including stiffness, slumping gait and confusion. Williams also had Lewy body dementia, which causes a progressive decline in mental abilities.
Several young people too kill themselves every day unable to cope with the physical and mental challenges life throws at them.
And unable to bear the huge medical expenses, it seems a few poor parents are requesting the courts here to give them the right to kill their severely ailing children.
Recently one of our Aunts died of multiple old age related diseases. She was 81 when she breathed her last and unable to bear her pain, she often used to say in the final stages of her life, "I cannot bear this any longer. I want to die. I daily pray to God that he should take me away from this world soon. The sooner the better". And immediately after her death, her relatives parroted the same words: "It is good to see her go from this world forever. She suffered a lot. Now she is free from all that suffering".
As a person of science and with the eternal hope my world brings, for me these stories and words are extremely disturbing. Is death the final solution to the suffering of people? No, no, no and a big NO is my scream. People should change this wrong way of thinking and their attitude. Where has the fighting spirit gone? Where has all the love for life gone? Where has the will to live gone? Where has the good will of comforting, supporting, helping and make people want to enjoy the boon of longer life given by science gone?
Homo sapiens were originally programmed to live for only a 25-35 years. Human beings and members of many other species necessarily experience ageing and mortality. In contrast, while a few animals like tortoises, some whales and fish live for longer periods, some other species can be considered immortal. For example, bacteria fission to produce daughter cells, perennial plants can produce clones of themselves by vegetative reproduction and are thus potentially immortal, and animals in the genus Hydra have a regenerative ability with which they avoid dying of old age.
Even within humans and other mortal species, there are arguably cells with the potential for immortality: cancer cells which have lost the ability to die such as the HeLa cell line, stem cells, and specifically germ cells (producing ova and spermatozoa). In artificial cloning, adult cells can be rejuvenated back to embryonic status and then used to grow a new tissue or animal without ageing. Normal human cells however die after about 50 cell divisions in laboratory culture. After a period of near perfect renewal (in humans, between 20 and 35 years of age), ageing is characterised by the declining ability to respond to stress, increasing homeostatic ( the property of a system in which variables are regulated so that internal conditions remain stable and relatively constant) imbalance and the increased risk of disease. This currently irreversible series of changes inevitably ends in death.
Modern science has been fighting this injustice done by Nature to human beings as compared to some other living beings and the time limit given to us and saw to it that it increased to 70-80 years now. So all the years you live after 35 is the bonus science gave the world. But ageing is among the greatest known risk factors for most human diseases.
In laboratory settings, researchers have demonstrated that selected alterations in specific genes can extend lifespan quite substantially in nematodes, less so in fruit flies and less again in mice. Some of the targeted genes have homologues (in the context of biology, homology is the existence of shared ancestry between a pair of structures, or genes, in different species) across species and in some cases have been associated with human longevity.
Ageing is usually defined as the slow accumulation of damage in our cells, organs and tissues, ultimately causing the physical transformations that we all recognise in elderly people. Biological theories of ageing in humans fall into two main categories. Programmed theories imply that ageing is regulated by biological clocks operating throughout the life span. This regulation would depend on changes in gene expression that affect the systems responsible for maintenance, repair and defense responses. Stochastic theories blame environmental impacts on living organisms that induce cumulative damage at various levels as the cause of ageing. Examples of environmental impacts range from damage to DNA, damage to tissues and cells by oxygen radicals (widely known as free radicals countered by the even more well known antioxidants), and cross-linking. However, ageing is now seen as a combination of genetic and environmental processes; a progressive failure of homeostatic mechanisms involving maintenance and repair genes, stochastic events leading to molecular damage and molecular heterogeneity, and chance events determining the probability of death.
The very essence of human life is the DNA molecule. When you look at the tips of the DNA molecule they contain a kind of chain of repeating pairs of enzymes. Called telomeres, these molecular chains have often been compared to the blank leaders on film and recording tape. Indeed, telomeres seem to perform a similar function. During the replication process the spiral DNA molecule must split in half and reassemble a copy of itself. Protecting the vital DNA molecule from being copied out of synch, telomeres provide a kind of buffer zone where mis-alignments (which are inevitable) will not result in any of the important DNA code being lost.
Telomeres Picture credit: Applied Health
As any cell - and our bodies are made of billions of these - gets older, it is under attack by oxides and free-radicals in the body and environment. We survive as living beings because our cells have the ability to duplicate and replace themselves before being killed by these natural causes. Each time our cells divide, the DNA molecule makes a new copy of itself. But the procedure is very complex and not perfect. Usually a small portion of the DNA molecule is lost, misaligned and not copied. Since errors are more frequent on the ends of the DNA molecule, this area, the telomere, does not contain any important DNA information and the effect is insignificant. Telomeres stabilize chromosomes and shortening of the telomeres after each cell division could cause instability.
Scientists observed that the length of telomere chains becomes shorter as we grow older. Eventually the telomeres become so short that cell replication produces lethal errors or missing pieces in the DNA sequence, ending the cell's ability to replace itself. This point, when the cell has lost vital DNA code and cannot reproduce, is called the Hayflick limit. It's the measure of how many times a cell can copy itself before it dies. Scientists have discovered an important enzyme that can turn the telomere production on the DNA molecule "on" and "off." It's called telomerase. Not surprisingly, it seems that as we get older, the amount of telomerase in our cells decreases and hence several errors occur leading to various age related health conditions.
Another in-built reason for diseases is malfunctioning of proteins. Proteins are huge molecules containing hundreds to thousands of atoms that adopt a unique three dimensional structure, placing chemical groups in just the right place to catalyze reactions or build cellular structures. Those atoms surprisingly usually manage to find the right location to fold. The complexity in the molecular 'wiring' of our genome--the way our proteins talk to each other--may simply be a side effect of a desperate attempt to stave off problematic random mutations in proteins' structures. Folding has important medical implications because most genetic defects cause protein misfolding. This loss of shape weakens the protein's ability to function. Proteins might be liable to spontaneously misfold--as they do in disorders such as Alzheimer's disease, Parkinson's disease and prion diseases, all of which are caused by misfolded proteins in the brain.
There are several other external and environmental factors too that effect the health of human beings like the Microbes, toxic pollutants.
Now that our understanding of what cause most diseases and how death occurs has increased drastically, science is trying to fight valiantly with all its strength all most all the unwanted conditions and to increase the life spans of people more. It is discovering new and potent medicines to treat them, coming up with correcting and supporting systems such as high precision and least invasive surgical operations and very effective physio-therapies using latest technologies, gene therapies and CRISPR-Cas–based genome editing procedures to correct disease causing mechanisms. Our battle is definitely to win, reduce the pain and suffering as much as possible, make lives worth living for longer periods.
Sometimes even young people develop diseases because of things gone wrong in their systems. Then will you say the same words you used for your old parents when your siblings, spouse or children are suffering? You may be busy and feel you have no time to look after your loved ones. If you cannot give love and comfort during their suffering, they lose all the enthusiasm to live and think of death as a solution to their problems. It is you who is responsible for their thinking in such a way not their condition actually. If you can spare some time for them, drown them in your love, empathize, understand and share their feelings, give psychological assistance, extend free medical services for the economically weak, provide the best medical care, they don't think drastically. They develop love for life despite their suffering. Co-operate with science in its endeavour of providing the best solutions to various problems people face. Don't ever make people lose all enthusiasm for life and think about killing themselves or inviting death as answer to their conditions. Don't make them so desperate.
Think about this, my dears.
Dr. Krishna Kumari Challa's art work based on the theme
Heroic Science
Views: 657
Replies to This Discussion
-
621
Our young cells survive only because they have a slew of trusty mechanics on call. Take DNA, which provides the all-important instructions for making proteins. Every time a cell divides, it makes a near-perfect copy of its three-billion-letter code. Copying mistakes happen frequently along the way, but we have specialised repair enzymes to fix them, like an automatic spellcheck. Proteins, too, are ever vulnerable. If it gets too hot, they twist into deviant shapes that keep them from working. But here again, we have a fixer: so-called ‘heat shock proteins’ that rush to the aid of their misfolded brethren. Our bodies are also regularly exposed to environmental poisons, such as the reactive and unstable ‘free radical’ molecules that come from the oxidisation of the air we breathe. Happily, our tissues are stocked with antioxidants and vitamins that neutralise this chemical damage. Time and time again, our cellular mechanics come to the rescue.
Which leads to the biologists’ longstanding conundrum: if our bodies are so well tuned, why, then, does everything eventually go to hell.
One theory is that it all boils down to the pressures of evolution. Humans reproduce early in life, well before ageing rears its ugly head. All of the repair mechanisms that are important in youth – the DNA editors, the heat shock proteins, the antioxidants – help the young survive until reproduction, and are therefore passed down to future generations. But problems that show up after we’re done reproducing cannot be weeded out by evolution. Hence, ageing.
Most scientists say that ageing is not caused by any one culprit but by the breakdown of many systems at once. Our sturdy DNA mechanics become less effective with age, meaning that our genetic code sees a gradual increase in mutations. Telomeres, the sequences of DNA that act as protective caps on the ends of our chromosomes, get shorter every year. Epigenetic messages, which help turn genes on and off, get corrupted with time. Heat shock proteins run down, leading to tangled protein clumps that muck up the smooth workings of a cell. Faced with all of this damage, our cells try to adjust by changing the way they metabolise nutrients and store energy. To ward off cancer, they even know how to shut themselves down. But eventually cells stop dividing and stop communicating with each other, triggering the decline we see from the outside. -
-
Aging: What underlies the mitochondrial stress response
Scientists at EPFL have discovered certain enzymes that play a central role in the stress responses that defend mitochondria from stress, and promote health and longevity.
Probably the most well-known organelle of the cell, mitochondria play a critical role in producing energy from food. So, it's no surprise that mitochondria can get stressed and damaged. When stressed, mitochondria turn on multiple defense mechanisms: biochemical "domino" pathways that help them repair their defects and recover or improve their health.
Stressed mitochondria are particularly linked to aging and many age-related diseases. Problematic mitochondria are the cause of diseases in metabolism, the cardiovascular and neuromuscular systems, and even certain cancers. Because of how central mitochondria are to survival and health, they have evolved multiple stress response pathways to adapt their function to the ever-changing environment of the cell. But how these stress responses are regulated is still largely unknown.
Now, a team of scientists has discovered that mitochondrial stress induces global but also very specific epigenetic changes, which involve enzymes that unravel compacted DNA in the cell's nucleus to activate genes. These enzymes are called histone acetyltransferases because they interact with the histone proteins that pack DNA into a structure called chromatin. The findings are published in Nature Aging.
Looking at the chromatin of the nematode C. elegans—a highly popular organism for studying aging—the scientists found that a histone acetyltransferase named CBP-1 is essential for the epigenetic changes caused by stress response of mitochondria, translating their stress signal into a coordinated transcription of a number of genes that are known to be involved in mitochondrial stress response.
The beneficial effects of the mitochondrial stress response, such as resistance to pathogen infections, improved proteostasis against amyloid-β aggregation—one of the culprits of Alzheimer's—and extending lifespan are almost completely dependent on these epigenetic changes. Moreover, analysis in mouse and human populations, as well as genetic and pharmacological loss-of-function studies in mammalian cells, strongly suggest that this epigenetic mechanism involved in the regulation of the stress response, health and lifespan is also conserved in the mouse and human.
This work identifies an evolutionarily conserved node for mitochondrial stress signaling that defends mitochondrial function, and promotes health and longevity. Drugs that target these mitochondrial stress pathways may be interesting to curb the aging process.
Terytty Yang Li et al. The transcriptional coactivator CBP/p300 is an evolutionarily conserved node that promotes longevity in response to mitochondrial stress, Nature Aging (2021). DOI: 10.1038/s43587-020-00025-z
https://phys.org/news/2021-02-aging-underlies-mitochondrial-stress-...
-
-
Researchers discover a new way to fight the aging process and cancer development
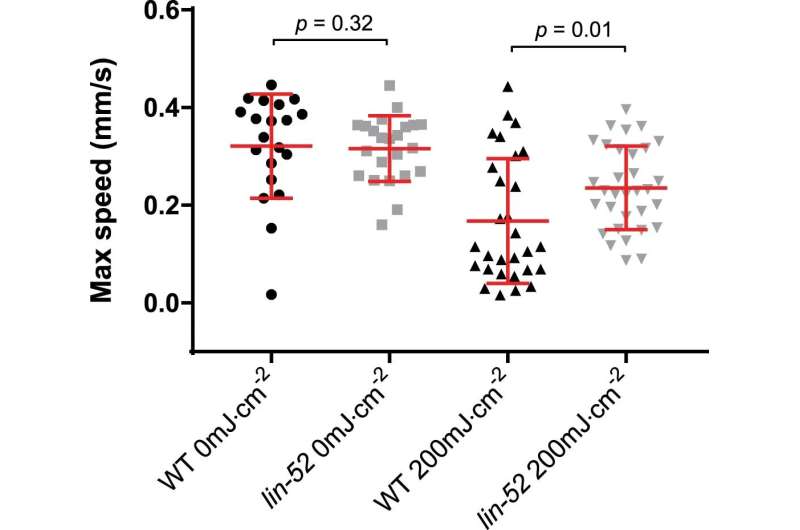 lin-52 mutant worms retained motility upon DNA damage induction. lin-52(n771) and WT day 1 adult worms were irradiated or mock-treated with UV-B and their motility was measured after 72 h. Maximum speed per individual is presented (mean +/− SD in red). Two-tailed Mann-Whitney test was used. From left to right, n = 20, 23, 29, 32. Credit: Nature Structural & Molecular Biology (2023). DOI: 10.1038/s41594-023-00942-8
lin-52 mutant worms retained motility upon DNA damage induction. lin-52(n771) and WT day 1 adult worms were irradiated or mock-treated with UV-B and their motility was measured after 72 h. Maximum speed per individual is presented (mean +/− SD in red). Two-tailed Mann-Whitney test was used. From left to right, n = 20, 23, 29, 32. Credit: Nature Structural & Molecular Biology (2023). DOI: 10.1038/s41594-023-00942-8
A protein complex prevents the repair of genome damage in human cells, in mice and in the nematode Caenorhabditis elegans, a team of researchers at the University of Cologne has discovered. They also successfully inhibited this complex for the first time using a pharmaceutical agent.
"When we suppress the so-called DREAM complex in body cells, various repair mechanisms kick in, making these cells extremely resilient towards all kinds of DNA damage," said Professor Dr. Björn Schumacher, Director of the Institute for Genome Stability in Aging and Disease at the University of Cologne's CECAD Cluster of Excellence in Aging Research.
Because it contains all of our genetic information, our DNA must be well protected. However, it constantly faces damage caused by environmental influences—or our normal metabolism. Hence, DNA repair is essential for the stability of our genome and the functioning of our cells.
"Our findings for the first time allow us to improve DNA repair in body cells and to target the causes of aging and cancer development," Schumacher added. Still, more research is needed until these results can be translated into new therapies for human patients. The study 'The DREAM complex functions as conserved master regulator of somatic DNA repair capacities' has appeared in Nature Structural & Molecular Biology.
DNA damage leads to aging and disease
Our genetic material is passed on from generation to generation. That is why it is particularly well protected in our germ cells. Highly precise DNA repair mechanisms are at work there, ensuring that only very few changes in the genetic material are passed on to offspring. Thanks to DNA repair, our human genome has been passed on to us by our ancestors for two hundred thousand years. It has always ensured that the genetic information is preserved. DNA is also constantly repaired in our body cells, but only for the duration of the individual's life.
Sometimes, children are born with faulty DNA repair systems, making them age more quickly and develop typical age-related diseases such as neuro-degradation and arteriosclerosis already in childhood. In some cases, they also have an extremely increased risk of cancer. These are all consequences of DNA damage not being properly repaired.
The DREAM complex prevents repairs
Schumacher and his team explored why body cells do not have the same repair mechanisms as germ cells. In experiments with the nematode C. elegans, they found out that the DREAM protein complex limits the quantity of DNA repair mechanisms in body cells: the complex attaches to the DNA's construction plans containing instructions for the repair mechanisms. This prevents them from being produced in large quantities.
Germ cells, however, do not have the DREAM complex. Hence, they naturally produce large quantities of DNA repair mechanisms.
Mammals also have a DREAM complex
In further experiments with human cells in the laboratory (cell culture), the scientists showed that the DREAM complex functions in the same way in human cells. They were also able to override the DREAM complex with a pharmaceutical agent.
"We were very pleased to see the same effect as we did in C. elegans. The human cells were much more resilient towards DNA damage after treatment," said Arturo Bujarrabal, a postdoc in Schumacher's team and lead author of the study. Treatment with the DREAM complex inhibitor also showed amazing effects in mice: The DNA in the retina of mice could be repaired and the function of the eye preserved.
The test was carried out in mice that, like some patients, age prematurely and show a typical degeneration of the eye's retina.
Genome damage also plays a major role in manned spaceflight because of the extremely high radiation in space. A longer stay in space without improved DNA repair is hardly imaginable. Schumacher says, "Therapies that target and improve this newly discovered master regulator of DNA repair could reduce the risk of cancer because genes remain intact."
In addition, the risk of age-related diseases would be reduced because cells can only fulfill their function with an intact genome.
More information: Björn Schumacher, The DREAM complex functions as conserved master regulator of somatic DNA-repair capacities, Nature Structural & Molecular Biology (2023). DOI: 10.1038/s41594-023-00942-8. www.nature.com/articles/s41594-023-00942-8
-
© 2025 Created by Dr. Krishna Kumari Challa.
Powered by
![]()